Chronic Inflammation as a Driver in MASLD: A Systems View
Hello everyone!
Welcome to my blog 🎉
Today, I will describe how metabolic dysfunction-associated steatotic liver disease (MASLD) arises and is sustained over time.
Chronic Inflammation Beneath MASLD
Metabolic dysfunction-associated steatotic liver disease (MASLD) is often framed as a disorder of lipid accumulation, insulin resistance, and disrupted energy homeostasis. Yet beneath these metabolic hallmarks lies a quieter but persistent force that shapes disease trajectory over years: chronic inflammation. Unlike the acute, self-resolving immune responses associated with infection, inflammation in MASLD is low-grade, sustained, and tightly interwoven with metabolic stress.
This form of inflammation does not announce itself dramatically. Instead, it stabilises, lingers, and gradually remodels tissue function, laying the groundwork for fibrosis and long-term liver injury.
Systems view
At a systems level, MASLD emerges from continuous dialogue between metabolic dysfunction and immune sensing. Excess nutrient availability, altered lipid species, mitochondrial stress, and oxidative damage generate intracellular danger signals within hepatocytes. These signals are not microbial, yet they are sufficient to disrupt immune tolerance.
Stressed hepatocytes release damage-associated molecular patterns (DAMPs), lipotoxic metabolites, and inflammatory mediators that signal compromised tissue homeostasis. In this context, the immune system is activated not to eliminate pathogens, but to respond to persistent metabolic imbalance.
Innate Immune Activation: Adaptation That Turns Maladaptive
The innate immune compartment is the first responder to metabolic stress. Liver-resident macrophages, together with recruited monocytes, sense danger signals through pattern-recognition receptors and inflammasome-associated pathways. Rather than triggering a rapid and resolving response, these cells enter a state of chronic activation.
Over time, this leads to sustained cytokine and chemokine production, reshaping the hepatic microenvironment and reinforcing immune cell recruitment. What initially functions as an adaptive response to stress gradually becomes maladaptive, perpetuating inflammation instead of restoring balance.
Engaging Adaptive Immunity: T Cells Enter the Circuit
Persistent innate immune activation does not remain isolated. Ongoing inflammatory signalling alters antigen presentation, co-stimulatory cues, and tissue architecture, creating conditions that engage the adaptive immune system. In particular, CD8⁺ T cells are recruited into a liver environment that is inflamed but not infected, metabolically altered but not acutely damaged.
In this setting, CD8⁺ T cells can produce cytokines and cytotoxic mediators that contribute to hepatocyte injury, even in the absence of classical antigenic triggers. This challenges conventional immunological frameworks and raises fundamental questions about how adaptive immunity behaves under chronic metabolic stress.
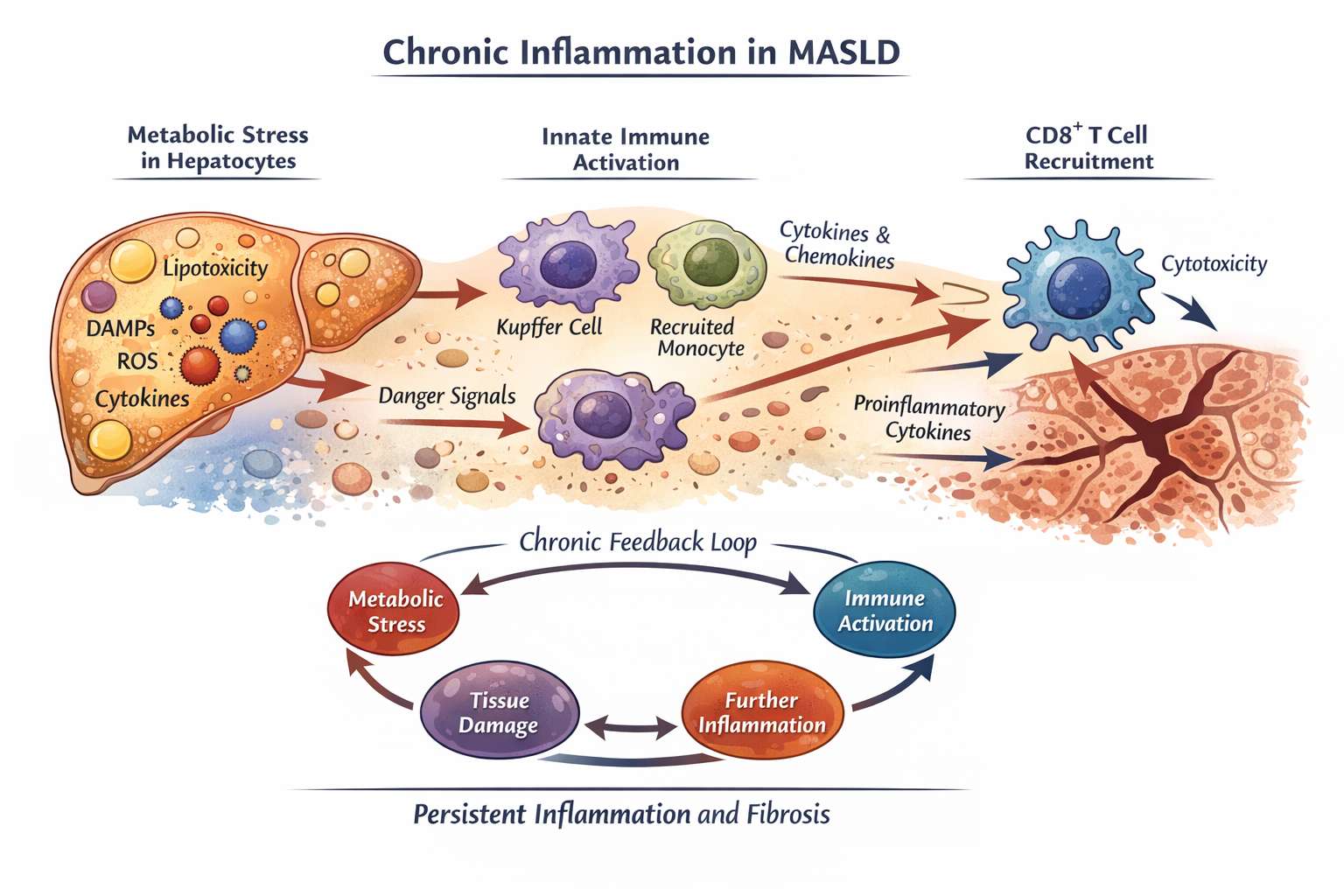
A Self-Sustaining Feedback Loop
Chronic immune activation in MASLD forms a reinforcing feedback loop:
Metabolic stress → innate immune activation → T cell recruitment → tissue injury → further immune activation
Once established, this loop becomes difficult to disrupt. Understanding where and how this cycle can be interrupted is central to identifying early intervention points and therapeutic strategies.
Open Questions Driving My Research
Several unresolved questions guide my work in this area:
How do T cell states differ between early MASLD and advanced fibrotic disease?
Which signals determine whether these cells adopt pathogenic versus regulatory roles?
How does chronic metabolic stress reshape T cell function across liver, adipose tissue, and circulation?
MASLD as a Model of Chronic, Non-Infectious Inflammation
Viewed through a systems lens, MASLD is not driven by a single cell type or pathway, but by interconnected feedback loops linking hepatocytes, immune cells, stromal populations, and metabolic cues. Inflammation amplifies metabolic dysfunction, which in turn sustains immune activation.
This reframes MASLD as more than a liver disease. It becomes a model for understanding how chronic, non-infectious inflammation emerges, stabilises, and ultimately drives tissue damage.